Ethylene Oxide (EtO) Development Support Document (DSD)
NEW The Ethylene Oxide Carcinogenic Dose-Response Assessment DSD has been finalized (dated May 15, 2020)
After its release in 2016, the TCEQ conducted a thorough scientific review of USEPA’s ethylene oxide (EtO) cancer dose-response assessment. TCEQ’s review identified serious scientific issues surrounding USEPA’s assessment. As a result, the TCEQ evaluated all relevant EtO data and conducted a cancer dose-response assessment. In doing so, the TCEQ was able to address the various scientific shortcomings of USEPA’s 2016 assessment and consider new data and analyses by the TCEQ or appearing in the scientific peer-reviewed literature since 2016.
Prior to conducting any assessments, as part of the TCEQ Guidelines to Develop Toxicity Factors, the TCEQ provides an opportunity to outside parties to submit any chemical-specific information they would like the Agency to consider. On August 16, 2017, the TCEQ publicly announced plans for the EtO assessment and requested any interested parties (industry, academia, NGOs, private citizens, etc.) provide any relevant information through our listserv and via our website. Any information submitted through this process is taken into consideration during the literature review phase of the assessment.
Subsequently, the TCEQ conducted extensive analyses for the EtO dose-response assessment; the results of the analyses guided the TCEQ’s decisions and approach to the assessment. The final Development Support Document (DSD) provides transparent documentation of those analyses, allowing readers the opportunity to see how and why decisions were made.
The first draft EtO DSD was proposed for public comment on June 28, 2019, and the public comment period ended September 26, 2019. The agency received numerous comments on the proposed DSD from diverse groups (industry, academia, NGOs, and private citizens). Scientifically substantive public commentswere reviewed and fully addressed by the TCEQ as documented in the Response to Public Comments(dated January 31, 2020).
Responding to public comments resulted in an improved
revised draft EtO DSD (dated January 31, 2020) that underwent an external expert peer review. The external scientific peer review was organized by the University of Cincinnati Risk Science Center and produced the
Final Report for Letter Peer Review(dated April 30, 2020).
(dated January 31, 2020) that underwent an external expert peer review. The external scientific peer review was organized by the University of Cincinnati Risk Science Center and produced the
Final Report for Letter Peer Review(dated April 30, 2020).
Scientific expert comments were thoroughly reviewed and addressed by the TCEQ as documented in the EtO DSD External Peer Review Response to Comments(dated May 15, 2020). The TCEQ responding to the external expert comments resulted in a state-of-the-science final EtO DSD(dated May 15, 2020).
- Both the TCEQ and USEPA final EtO cancer dose-response assessments are ultimately based on the same NIOSH cohort of workers; results from those workers have been adjusted for the general U.S. population.
- The TCEQ cancer dose-response assessment is based on the same carcinogenic mode of action (MOA) as USEPA’s assessment (i.e., a mutagenic MOA); consequently, TCEQ’s assessment does not assume a threshold but rather that carcinogenic risk may be estimated no matter how small the EtO inhalation dose, all the way down to zero dose.
- In the TCEQ’s assessment, adjustment for the greater susceptibility of children was conducted by applying age-dependent adjustment factors (ADAFs) using USEPA’s preferred method.
- The USEPA acknowledges that human data are insufficient to demonstrate that EtO causes any cancer in humans (e.g., lymphoid or breast cancer), even in workers exposed to levels up to millions of times higher than environmental levels to which the general U.S. population may be exposed. Nevertheless, the TCEQ assessment evaluates both lymphoid cancer and breast cancer as candidate endpoints, ultimately utilizing lymphoid cancer as the cancer endpoint with the strongest (although still insufficient) human evidence.
- The TCEQ assesses lymphoid cancer risk to both males alone and males + females combined. However, because males appear more susceptible than females to EtO-induced lymphoid cancer, it is important to understand that TCEQ’s application of male dose-response results to the general population (including females) results in a lower health-based air concentration for everyone (i.e., the health-based air concentration would actually be higher if male + female results were used).
- The USEPA used an unconventional and overall supra-linear model that is statistically significantly over-predictive of the lymphoid cancer data when compared to the data that was used to develop the model. USEPA’s model selection process was flawed because the agency misinterpreted Science Advisory Board advice on model parameter values and miscalculated model fit criteria (e.g., Akaike information criteria (AIC) and model fit p-values). USEPA’s error was estimating model parameter values (i.e., “knots”) based on model fit to the data and then not counting them as fitted values in the AIC and p-value calculations.
- The TCEQ used a widely accepted dose-response model, the Cox proportional hazards model. This standard dose-response model neither statistically over- or under-estimates the number of lymphoid cancer mortalities when compared to the data used to develop the model, but rather is reasonably accurate; the same cannot be said for USEPA’s model. Furthermore, the AIC and p-value model fit criteria calculated by the TCEQ support using the widely accepted Cox proportional hazards model over the unconventional model used by USEPA. The Cox proportional hazards model has a lower/better AIC for model fit (low AIC is an indication of better model fit), and the TCEQ’s selection of the Cox proportional hazards model is also strongly supported by other considerations, such as the model predictions mentioned above.
- It is important to note that the human body naturally produces appreciable levels of EtO (a.k.a. endogenous EtO). In contrast to TCEQ’s risk-based results, USEPA’s risk-based air concentrations correspond to doses that are orders of magnitude below even the 1st percentile of the normal endogenous range of EtO in the nonsmoking population. In other words, the human body naturally produces EtO at levels that are orders of magnitude higher than doses corresponding to USEPA’s calculated risk-based air concentrations. As a result, USEPA’s assessment appears to overpredict the actual measured rate of lymphoid cancers in the general U.S. population.
- Some public comments suggest, because of the “Healthy Worker Effect” (workers may be healthier than the general public and therefore may be less sensitive to the effects of chemicals), that the TCEQ’s model prediction calculations of the lymphoid cancer data used to develop the models are not appropriate. However, the 95% confidence interval (CI) on the Standard Mortality Ratio (SMR) for unexposed NIOSH workers includes 1, which indicates that the mortality rate in the unexposed workers and the U.S. population mortality rate are not statistically significantly different. Similar results are obtained for the male NIOSH workers that drive lymphoid cancer risk (i.e., the lymphoid cancer SMR in unexposed NIOSH males is 1.03 (6/5.8) with a 95% CI of 0.38, 2.25). Thus, it is demonstrated that in fact, there is no Healthy Worker Effect for this critical cancer endpoint in this key cohort (i.e., TCEQ calculations are appropriate).
I have input/comment from Toxicologist Elena Craft, an Austin-based toxicologist who is Environmental Defense Fund’s senior director for climate and health. Below is the comment I have from her, communicated last week. I wish to extend the opportunity to the agency to offer comment/response to her comment she offered as it relates to proposed efforts to revise a cancer risk assessment for ethylene oxide, public comment period ending Sept. 26.
Comments by the EDF toxicologist Elena Craft:
“We will have more on this soon, but just taking an initial look at some of material that TCEQ is using to assess threshold values of EtO, there are real problems with the data that they are relying on for their assessment, specifically the work from Kirman and Hays.
“To summarize, TCEQ has done a meta-analysis using disparate data sets from disparate countries around the world to account for EtO exposure. What you have is a very mixed bag of people, many of whom have not been properly controlled for potential occupational exposures.
“TCEQ’s criteria for inclusion in the meta-analysis are very loose and there is no sensitivity analysis to see what the impact might be of dropping some folks (i.e., Do your conclusions still hold if remove datasets that may not have been properly controlled?).
“It is also clear that there is a specific phenotype in some people that is more active/sensitive to hemoglobin adducts, which is the biomarker that they are using for exposure.
“If there is a difference across study populations in the percentage with this phenotype, it could make these studies non-comparable, which would make their meta-analysis irrelevant. It also seems that they are trying to force the data to fit into the model that they are using.
“Overall, we have very little confidence in the assessment of data put forward by TCEQ on a compound that is contributing to some of the largest cancer risks in people across the nation.”
Dr. Craft seems to be confusing the TCEQ’s ethylene oxide (EO) dose-response assessment with one of the peer-reviewed papers that was referenced in our document (Kirman and Hayes, 2017). In contrast to Dr. Craft’s characterization, the TCEQ conducted a non-threshold assessment that uses the same data that EPA used from a United States-based group of workers to calculate air concentrations of EO that are expected to cause minimal cancer risk for the general public. The information from the Kirman and Hayes (2017) study was not used to calculate the final EO cancer risks, but rather was used to put risk results into context. We confirmed the validity of the standard mathematical method we utilized by demonstrating that our selected model assessment could accurately reproduce the cancer mortality data in the group of US workers (EPA’s model did a poor job of predicting the cancer risks in the group of US workers). This validation demonstrates that TCEQ chose an appropriate model for determining the cancer risks of exposure to EO. We appreciate Dr. Craft taking the time to provide comments on our assessment, and we welcome any and all further comments. More detailed responses to Dr. Craft’s comments are included below.
Dr. Craft is incorrect in many of the statements she makes about our assessment, including:
1. That we derived threshold values for EO;
2. That we used the Kirman and Hayes (2017) analysis as the basis for our risk-based value for EO;
3. That we conducted a meta-analysis;
4. That we used data-sets from around the world;
5. That we used EO-hemoglobin adducts as a biomarker of exposure for dose-response modeling; and
6. That we forced the data to fit the model.
Dr. Craft focuses on the Kirman and Hayes (2017) work at the beginning of her comments, so perhaps she was confusing their work (the purpose of which was to determine endogenous EO levels in the body), with our carcinogenic dose-response assessment of EO.
The following is our clarification of Dr. Craft’s misrepresentations:
1. The TCEQ is not conducting a threshold assessment or deriving threshold values, but rather is assuming cancer risk all the way down to zero dose with no threshold.
2. The work of Kirman and Hayes (2017) was used as supporting information, along with dozens of other studies and other lines of evidence (e.g. background EO concentrations, mutagenicity, epidemiological analysis, animal studies, etc). In particular Kirman and Hayes provided information about how much EO the body normally produces. This supporting information was not the basis of our derived EO risk value, but rather was used to provide context for our and EPA’s EO values.
3. We did not conduct a meta-analysis. The TCEQ uses the same NIOSH worker cohort study data as was used by the EPA to derive non-threshold risk-based values for lymphoid cancer caused by EO.
4. The datasets that the TCEQ assessed for deriving risk-based values for EO were 2 United States-based worker cohort studies: the NIOSH cohort (which both the TCEQ and the EPA ultimately used to derive EO values) and the Union Carbide cohort (UCC).
5. The TCEQ did not use EO-hemoglobin adducts as a biomarker of EO exposure for our dose-response modeling. We used the exposures estimated by the NIOSH and UCC study authors, which was based on measured and modeled EO air concentrations.
6. Not only did the TCEQ not force the cohort data to fit our dose-response model, we actually demonstrate mathematically that our model fits the data well, and our model fits the data much better than EPA’s model does. See Figures 8 through 12 on pages 42-46 of the Development Support Document. Because our model fits the data well, and better than EPA’s model, our model can better estimate ethylene oxide risk to the public.
Our document demonstrates that our assessment is more accurate than EPA's and is supported by multiple and convincing lines of scientific evidence (e.g., considerations of biological plausibility, reality checks on background incidence, model fit to the data, etc.).
Because ethylene oxide is emitted in Texas and has been determined to be a carcinogen, TCEQ undertook an inhalation carcinogenic dose-response assessment to derive a unit risk factor and an effect screening level for this chemical. These numbers are not alternatives to EPA’s IRIS value; they are derived by TCEQ to be used in the TCEQ air permitting program. TCEQ has similarly derived toxicity factors for thousands of chemicals for use in Texas programs (for more information: https://www.tceq.texas.gov/toxicology/esl).
TCEQ bases its ethylene oxide number on the same studies that the EPA uses. However, the primary difference between the two assessments is that the TCEQ uses a different modeling method for estimating cancer risks from that data. TCEQ has shown the EPA assessment, which rejects the standard Cox proportional hazards model in favor of an unconventional overall supra-linear model, to be scientifically flawed. For example, not only does the EPA assessment rely on incorrectly calculated p-values and Akaike’s Information Criteria values in selection of their model (discussed in TCEQ’s assessment), but TCEQ has demonstrated the EPA model to be over-predictive of the very lymphoid cancer data that drives EPA’s unit risk factor (described below). The EPA’s Science Advisory Board did not specifically review the issue about the p-values and AIC values that the EPA calculated, but the EPA’s calculations were inconsistent with the SAB’s comments on fixed parameters not estimated from the data, and the SAB has never reviewed TCEQ’s calculations of p-values or Akaike’s Information Criteria values. Furthermore, EPA’s maximum acceptable concentration corresponds to a dose that is about 1/40th of even the 1st percentile of the distribution of ethylene oxide doses that your body naturally produces (endogenous); such a miniscule additive dose is not biologically plausible to have actual significance given the range of endogenous doses.
TCEQ assessment will undergo independent external expert peer review in the next six months.
TCEQ’s toxicity factor derivation guidelines do not require that we use an IRIS value. Our guidelines indicate that we should review existing toxicity factors to determine if the methods etc. used are similar to the methods that the TCEQ would use to conduct an assessment. If the assessment is recent and the methods are sound TCEQ can consider adoption of an existing toxicity factor. However, as is described herein TCEQ did not find the EPA’s assessment methodology for the ethylene oxide derivation to be sound, and therefore we did not adopt the EPA’s number. TCEQ’s assessment, analyses, and rationales are plainly laid out in the DSD for any member of the public or media to review. TCEQ appears to have derived a weaker limit than EPA by eliminating breast cancer risks in its assessment. TCEQ also threw out the EPA’s conclusion that cancer risks from ethylene oxide increase dramatically at lower levels of exposure and flatten out at higher concentrations. Instead, TCEQ says, the carcinogenic chemical is only dangerous at higher levels of exposure. The commission’s scientists further undercut the EPA’s safety limit by discounting childhood exposure when calculating lifetime cancer risks. The practical effect is every judgment TCEQ made regarding the science is weighted toward industry. How does this protect public health?
TCEQ makes judgments based on the weight of scientific evidence. The weight of evidence for ethylene oxide-induced breast cancer is detailed in the proposed assessment and will be further detailed in the version revised after public and peer review comments.
The choice of how to estimate the change in cancer risks with increasing concentrations of a chemical depend on how the chemical does damage to the body (called the mode of action). Ethylene oxide directly interacts with DNA to cause damage that can lead to mutations and cancer. TCEQ and the EPA agree that this is ethylene oxide’s mode of action. TCEQ then used the standard approach for a chemical with this type of mode of action, which predicts a linear increase in cancer risks with increasing ethylene oxide exposure. You are correct that this method assumes that only higher levels of the chemical are dangerous, which is consistent with the vast majority of substances in our lives. The EPA’s approach of assuming that lower concentrations have higher risk (called a supra-linear model) is not consistent with ethylene oxide’s mode of action, nor could EPA provide any justification for using that type of concentration model based on ethylene oxide mechanistic information. In fact, the EPA’s SAB advised the EPA to use a model with a dose-response form that is both biologically plausible and consistent with the observed data. However, the EPA does not provide biological plausibility (i.e. mode of action) for a supra-linear model, nor does that model accurately predict the observed data (as discussed in our DSD and in responses to questions below).
In no place in the DSD does TCEQ discount childhood exposure (i.e., EPA age-dependent adjustment factors for childhood exposure are applied to the values), and it is not clear to us where you got this information.
Public health is best protected using the best available science. That is, science that realistically predicts risk so that priorities to mitigate exposures are properly set and efforts are expended on reducing exposures to chemicals commensurate with the risks that they truly represent to public health. The primary benefactors of decisions based on sound science are ultimately the public. Based on TCEQ’s ethylene oxide assessment, public health can be protected both against unacceptable environmental levels, and with the use of ethylene oxide-sterilized medical devices. TCEQ is on the side of the best available science.
TCEQ initially proposed a limit for ethylene oxide that was 65 times weaker than the IRIS value. The current proposal is 3,500 times weaker than the IRIS value. A memo published by USEPA discounts virtually everything in the TCEQ assessment, concluding that the only scientifically plausible alternatives to the IRIS value would be 2 to 3 times weaker. How does TCEQ justify such a dramatic change in its own assessment and a limit so radically different than EPA’s? The movement from a limit 65 to 3,500 times weaker occurred after TCEQ met with the American Chemistry Council. What influence did the ACC have on the commission’s proposal?
When EPA IRIS toxicity factors or toxicity factors from other agencies are promulgated, the TCEQ performs a quick, expedited review of them to determine if we want to adopt those values, or alternative values, on an interim basis. At the time (March 2017) TCEQ adopted an interim animal data-based unit risk factor for ethylene oxide to be used until a thorough multi-year systematic review and dose-response assessment could be conducted under the extensive TCEQ toxicity factor guidelines. As part of TCEQ’s standard process for conducting a thorough review of the literature for our ethylene oxide cancer dose-response assessment, we sent out a request for ethylene oxide information on Aug. 16, 2017. In response to the request for information, any interested parties (e.g., industry, academia, NGOs, private citizens) can submit whatever they think is relevant information (e.g., studies, presentations) for the agency’s evaluation. Such parties can request a meeting with TCEQ to present information, such as the American Chemistry Council (ACC) did. Similarly, after the TCEQ released the draft DSD for public comment, the Environmental Defense Fund requested and received a meeting with TCEQ to discuss their comments. While the agency greatly appreciates the submittal of information and comments by all parties, we ultimately evaluate and weigh all relevant scientific information on our own and based on our guidelines to arrive at our own decisions. TCEQ has conducted extensive analyses of multiple lines of evidence, each of which could have either supported a given approach or not. The results of each set of analyses have guided the TCEQ’s decisions and approach, as is clearly and transparently documented in the scientifically reasoned DSD.
We are assuming that you are referencing the Oct. 18, 2019, memorandum from Kristina Thayer of the EPA’s Office of Research and Development. That memo does not refer to Texas or TCEQ at all, and it describes the modeling choices that the EPA made in their 2016 ethylene oxide assessment. However, the EPA reiterating their modeling conclusions does not provide any additional evidence that those conclusions are correct. The TCEQ has shown in our DSD that the EPA assessment, which rejects the standard Cox proportional hazards model in favor of an unconventional overall supra-linear model, is scientifically flawed (e.g., see the first response and TCEQ’s document).
In a June 18 email to Erin Chancellor, TCEQ’s Michael Honeycutt said the agency’s modeling found that EPA had vastly overestimated lymphoid cancer risks— predicting 1,179 workers developed cancer when only 53 actually did. “It’s kind of hard to argue about the math,” Honeycutt wrote. Yet when TCEQ released its proposal 10 days later, the criticism of EPA’s modeling was significantly dialed back, predicting 141 cases instead of 1,179. How is that “over protective?”
The proposed DSD does not indicate that the IRIS factor overestimates by 1,179 deaths because that figure was an interim placeholder figure while documentation of the relationship between the upper and lower splines of EPA’s two-piece spline model could be located in EPA’s document. It was known at the time that the EPA model overestimated but not by exactly how much. As detailed in the document, TCEQ has shown EPA’s selected model assessment to be over-predictive for lymphoid cancer in the NIOSH cohort as a whole (comparing the predicted 141 cancer cases to the actual 53 cancer cases), for every exposure group, and for the US general population (when factoring in endogenous production of EtO).
The TCEQ document claims naturally occurring EtO exceeds the risk value calculated by EPA. But IRIS document clearly states the risk level is for exposures above background. In other words, from exposure to ethylene oxide pollution. Why is TCEQ repeating these arguments debunked by the EPA and the SAB?
The human body cannot tell the difference between ethylene oxide that is produced by industrial sources, versus small amounts that are naturally present in the air, versus the ethylene oxide that is produced internally by our own metabolism. It is the total amount of ethylene oxide that comes into contact with the DNA in cells that will lead to an increase in cancer, regardless of the source – this is a basic toxicological principle that states that equal internal doses give rise to equal risk.
The EPA’s 2016 dose-response assessment was based on occupational exposures of workers who, let’s assume, all had the same low background exposure to ethylene oxide (from ambient air and internal production). Therefore, as EPA states, the risk estimates produced by those studies would be the risk caused by occupational exposure to ethylene oxide in addition to the background exposure. From analysis of this data, the EPA ultimately concludes in their dose-response assessment that air concentrations higher than 10 ppt will cause an unreasonably high cancer risk (higher than 1 in 10,000 excess risk). However, the EPA has recently measured ethylene oxide concentrations of 110-220 ppt in areas with no nearby ethylene oxide sources. Based on the conclusion about risk in addition to background, one could conclude then that the EPA would choose an acceptable air concentration of 110-220 + 10 ppt (let’s say 120 ppt). However, the EPA does not do this, they use 10 ppt as the acceptable concentration limit, meaning that they are not considering risk levels above background.
As noted in our DSD, estimated average internally-produced concentrations of ethylene oxide are about 1.9 ppb in non-smokers and about 18.8 ppb in smokers. Accordingly, at measured background levels of ethylene oxide, the EPA’s unit risk factor for lymphoid cancer (7.1E-03 per ppb, ADAF adjusted) would predict a population-weighted lymphoid cancer incidence rate of ≈3.7% (in the absence of any exogenous ethylene oxide ambient air exposure or other potential causes of lymphoid cancer). By contrast, the EPA-cited lymphoid cancer background incidence rate (which would have many contributing factors, not just a single chemical) is 3%, demonstrating that EPA’s unit risk factor overestimates observable lymphoid cancer risk based on endogenous/background levels of ethylene oxide alone.
What are the “unintended societal consequences” TCEQ repeatedly refers to in its proposed limit for ethylene oxide?
Misinformed and misplaced environmental priorities; medical device shortages https://www.fda.gov/news-events/press-announcements/statement-concerns-medical-device-availability-due-certain-sterilization-facility-closures
After the Sept. 26 deadline, TCEQ will consider the public comments, revise the assessment as needed, and then post the assessment as final, along with responses to comments. Once finalized, TCEQ will use the ethylene oxide effect screening level in the review of air permits for new facilities in Texas. The newly derived ethylene oxide long-term ESL will replace the one that TCEQ is currently using, which is set at 1 part per billion. TCEQ’s proposed long-term ESL is 4 ppb.
I know it says so on the proposal, but I was having a hard time summarizing exactly why the TCEQ concluded that the EPA had over-estimated the cancer potency of ethylene oxide due to what the agency called their use of an improperly validated, unconventional mathematical model?
To derive the ethylene oxide cancer dose-response assessments, both EPA and TCEQ used data from a United States-based group of workers who were exposed to very high concentrations of ethylene oxide for many years and who experienced an increased rate of lymphoid cancers. From this data, both TCEQ and EPA had to estimate what the risk would be to a person who was exposed to typical environmental concentrations of ethylene oxide, which can be millions of times lower than the occupational levels the workers had been exposed to. The first step in this extrapolation is to determine how the chemical could cause cancer: In this case, ethylene oxide can cause cancer by causing damage to DNA. Based on that mechanism, the standard and conventional risk assessment method is to use a mathematical dose-response model that essentially draws a best-fitting straight line from the high dose data (from the worker exposure study) down to low doses (so it is applicable to ambient exposures). This is the standard method that TCEQ used, and using that method, agency toxicologists were able to accurately predict the number of cancers that were observed in the worker study. In contrast, instead of using the standard straight-line risk model, the EPA chose to assume that low doses of ethylene oxide are more potent than high doses for causing cancer (this is called a supra-linear model, and is the unconventional model that TCEQ referred to). EPA’s model was shown by TCEQ to significantly over-predict the number of cancers that were observed in the worker study, which is how we mathematically demonstrate that the EPA’s method over-predicts cancer risk.
In addition, the human body naturally produces low levels of ethylene oxide, with background levels being higher in smokers. Using the EPA’s risk assessment, the background levels of ethylene oxide in the population would be predicted to cause more lymphoid cancer than is actually observed in the general population (and ignoring any other potential cause of lymphoid cancer). In this way, we also know that EPA’s model over-estimates the cancer potency of ethylene oxide.
Potentially, how much ethylene oxide petrochemical plants would be able to emit if proposal goes through?
If finalized, this new effect screening level for ethylene oxide to be used in air permitting of new facilities would be similar to the one that TCEQ is currently using (see the response to your first question).
Why is TCEQ’s assessment more accurate than that of the EPA?
As noted in the response to your second question, TCEQ’s model accurately predicts the lymphoma cancer risk that was documented in the underlying U.S. worker study, while the EPA’s model was mathematically demonstrated by TCEQ to significantly over-predict risk.
Some of the concerns expressed by environmental groups include, that the goal is to “turning back the clock on progress for public health protection”; that using the TCEQ’s value instead of the IRIS value would likely lead TCEQ to try to ignore health threats from all EtO-emitting facilities, including chemical and petrochemical plants in TX. And that TCEQ appears to ignore the breast cancer research, that It focuses on a males-only occupational study to set its value, which is based on lymphoid cancer risk.
True, progress in the protection of public health cannot be made based on demonstrably flawed science but rather must be made on accurate assessments of the risks posed by chemicals. Only then can chemical exposures of the public be appropriately prioritized for mitigation to achieve the greatest reductions in real health risk. Otherwise, public, government, and industry efforts and resources are wasted on addressing unrealistic risks created by flawed science. The TCEQ assessment, which more accurately predicts cancer risk than EPA’s (as discussed above), will allow Texas to better assess potential health risks posed by ethylene oxide-emitting facilities in Texas and act accordingly. The TCEQ assessment is not based on a males-only study but rather the same NIOSH cohort used by EPA, which is composed of about half female workers.
The TCEQ assessment does evaluate breast cancer as a potential endpoint, and agency toxicologists anticipate that even more information on breast cancer risk will be included in the final assessment document. Lymphoid cancer, however, is the primary endpoint in TCEQ’s assessment and the primary contributor to ethylene oxide risk in EPA’s assessment. Based on the dose-response analyses for lymphoid cancer, females appear to have less risk from ethylene oxide exposure than males. Therefore, using risk results based on males results in even greater protection for females.
The EPA said the greatest is for people who have lived near a facility releasing EtO into the air for their entire lifetime. And that for a single year of exposure to ethylene oxide, the cancer risk is greater for children than for adults. This is because ethylene oxide can damage DNA. For everyone, including children, risks would decrease with decreased exposure. Does TCEQ’s assessment take this into account, especially given the number of facilities in the state?
Yes, risks are greatest for people who are exposed to EtO for their entire lifetime, and lifetime risks are greater when exposure begins in childhood. To ensure that children are also protected, the EPA and TCEQ both use age adjustment factors.
What could be the consequences of what TCEQ considers an overestimation of risk by the EPA?
Please refer you to TCEQ’s response to the fifth question, as well as to the FDA: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-steps-agency-taking-prevent-potential-medical-device
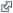
What are current guidelines being applied in Texas?
The current long-term value for ethylene oxide is 1 ppb, which is an interim value intended to be conservative until a fully scientifically rigorous assessment with extensive dose-response analyses could be conducted. By TCEQ having done so, such an assessment fully supports the proposed value of 4 ppb.
When is the last time TCEQ disagreed with an EPA assessment? Is this unusual?
TCEQ works with EPA daily on a wide range of issues. TCEQ and EPA typically agree on issues, but sometimes do not. It’s not unusual for states to disagree with EPA. However, in this case, TCEQ demonstrated that its assessment more accurately predicts the cancer potency of ethylene oxide than the EPA’s assessment (see answers given above).
Do you have a different proposal or guideline for workers?
TCEQ does not regulate occupational exposures. You may try contacting the Occupational Safety and Health Administration.
The TCEQ’s proposal comes at a time when other states, starting with Illinois, are moving to enact stricter standards. Any thoughts on that?
TCEQ began considering its currently proposed ethylene oxide assessment in early 2017 and put out a public request for information on Aug. 16, 2017. It is the agency’s understanding that EPA collected the initial ethylene oxide air samples around the Willowbrook, Ill., facility in May 2018. This May 2018 EPA sampling event was the beginning of Illinois’ (and other state’s) ethylene oxide activities.
Yes, EtO is produced endogenously in the body due to oxidation of ethylene.
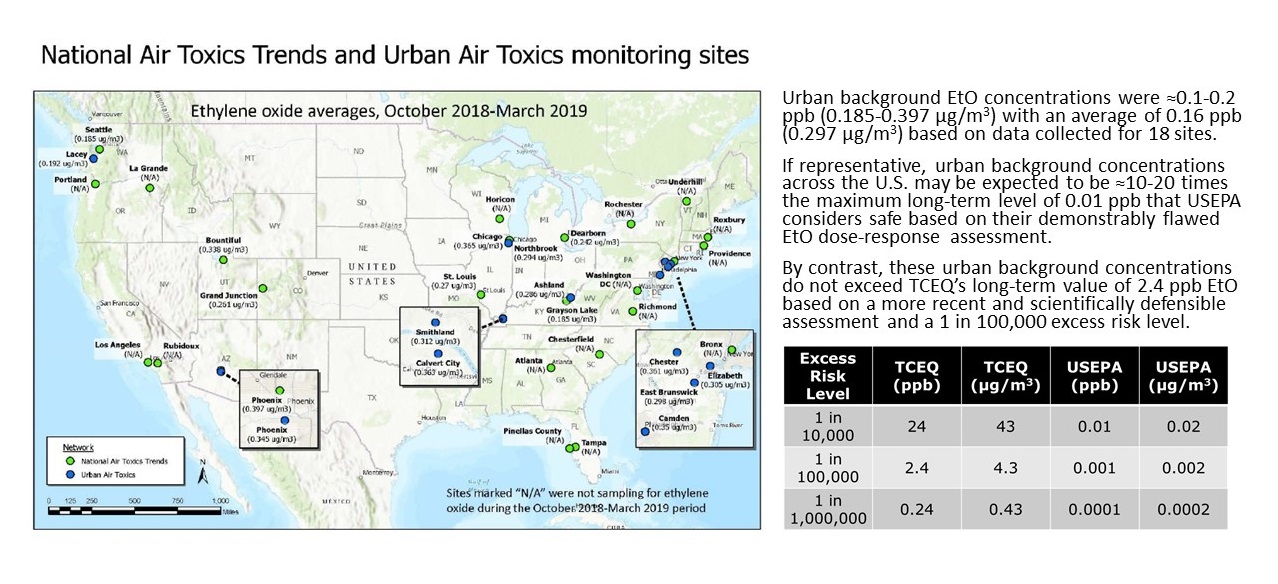
The range of EtO naturally present in the human body is equivalent to continuous exposure to approximately 0.56-4.5 ppb in air, with a mean of approximately 1.9 ppb compared to typical urban air background concentrations of approximately 0.1-0.2 ppb.
No, EtO has not been conclusively demonstrated to cause cancer in people. The United States Environmental Protection Agency (USEPA) and the Texas Commission on Environmental Quality (TCEQ) agree that human data are insufficient to classify EtO as a known human carcinogen. In workers exposed to concentrations millions of times higher than EtO levels the general public is exposed to, some studies show an association with increased cancer risk while others do not. Human evidence appears strongest for lymphoid cancer, although still inadequately strong. While some animals exposed to even higher EtO concentrations developed certain cancers, these data are of highly questionable relevance to humans. Despite the inconclusive evidence in people, both the TCEQ and USEPA have chosen to assume that EtO causes cancer in people and derive cancer-based toxicity factors to protect the public from the potential carcinogenic effects of long-term EtO exposure.
No, both assessments use the NIOSH cohort of over 17,000 workers. The major difference between the assessments is that the TCEQ chose a standard dose-response model (Cox proportional hazards model) supported by the mode of action by which EtO may cause cancer as well as other important considerations while USEPA chose an unconventional, overall supra-linear model for which they acknowledge there is no mode of action support.
Yes, the TCEQ has demonstrated its dose-response assessment to accurately predict the actual number of lymphoid cancers that occurred in the key NIOSH study population, while USEPA’s model is demonstrated to be statistically significantly over-predictive of the cancers that occurred in that population. This figure below shows TCEQ’s model to be accurate, while USEPA’s model statistically significantly over-predicts the number of actual cancer.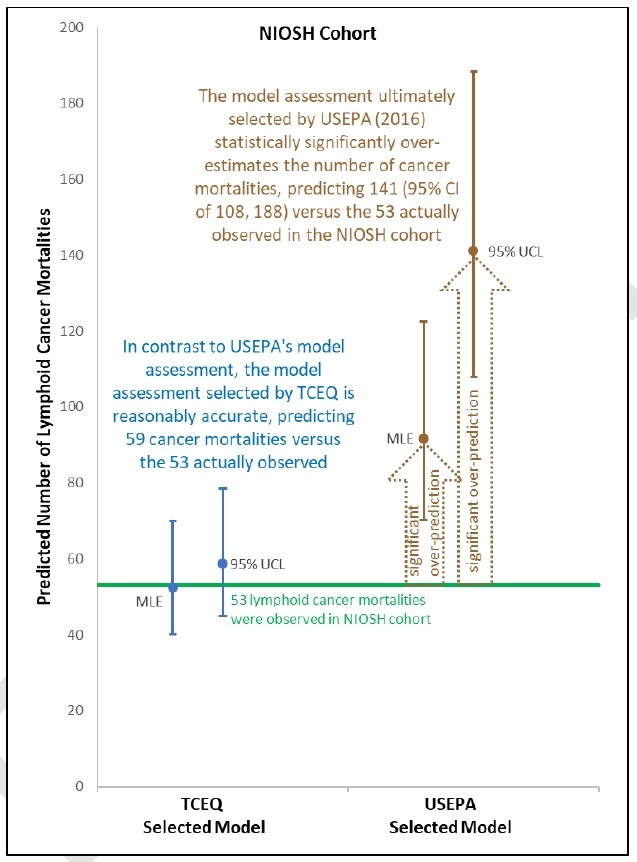
No, but the statistically significant over-prediction demonstrated by the TCEQ above for USEPA’s second choice model would have been even worse had USEPA selected their best fitting model (e.g., best estimate of 107.78 lymphoid cancers compared to the 53 observed). In large part, USEPA chose over-predictive models because of their incorrectly calculated model fit criteria (see the
TCEQ DSD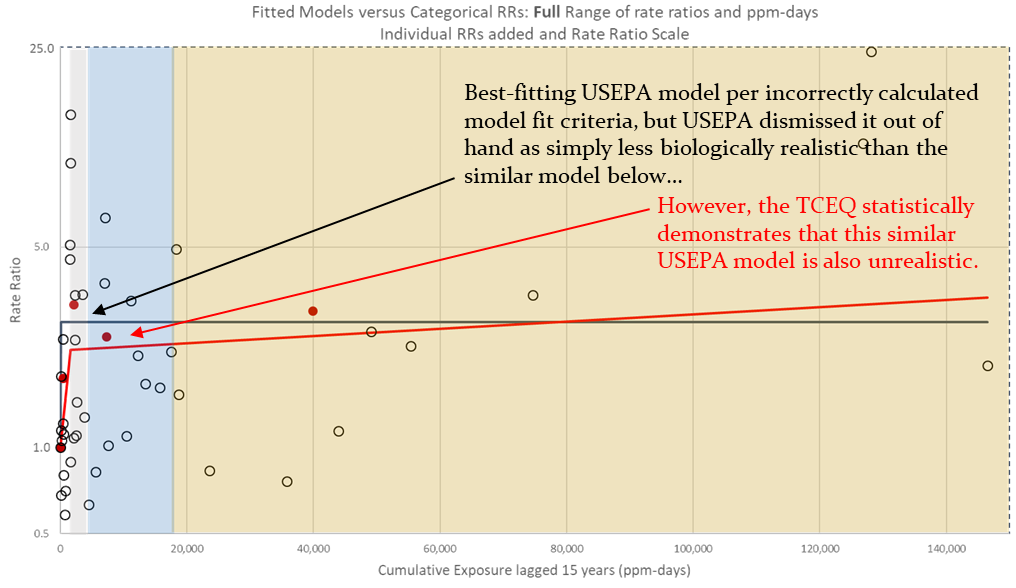 .
.
No, the TCEQ scientifically demonstrated:
(1) No significant difference in lymphoid cancer between the unexposed workers and the general U.S. population (i.e., no healthy worker effect);
(2) Robust epidemiological data published in the peer-reviewed literature and collected in workers specifically indicate the lack of a healthy worker effect for this endpoint (Kirkeleit et al. 2013); and
(3) Even assuming a healthy worker effect, USEPA’s model still significantly over-estimates the cancers observed, while TCEQ’s model neither over- or under-estimates the number of observed cancers.
Yes, the TCEQ assessment considers EtO as a potent carcinogen, estimating its potency as similar to benzene. Benzene is a carcinogen for which human data do conclusively demonstrate that it causes cancer in humans (unlike EtO). Furthermore, since EtO has a mutagenic mode of action, the TCEQ uses USEPA’s age-dependent adjustment factors to account for early-life exposure to EtO and the greater potential sensitivity of children.
No, the SAB did not specifically review every important aspect of the final USEPA assessment (e.g., model fit criteria calculations). Additionally, since USEPA’s review, new studies have been published that provide important information not available to SAB at that time (e.g., Vincent et al. 2019, Marsh et al. 2019, IARC 2019, Kirman and Hays 2017).
Yes, the TCEQ dose-response assessment considers new data and analyses. The scientific weight of evidence clearly indicates that TCEQ’s assessment conducted using the standard Cox proportional hazards model results in more reliable and reasonable estimates of excess risk than USEPA’s unconventional, overall supra-linear two-piece spline model.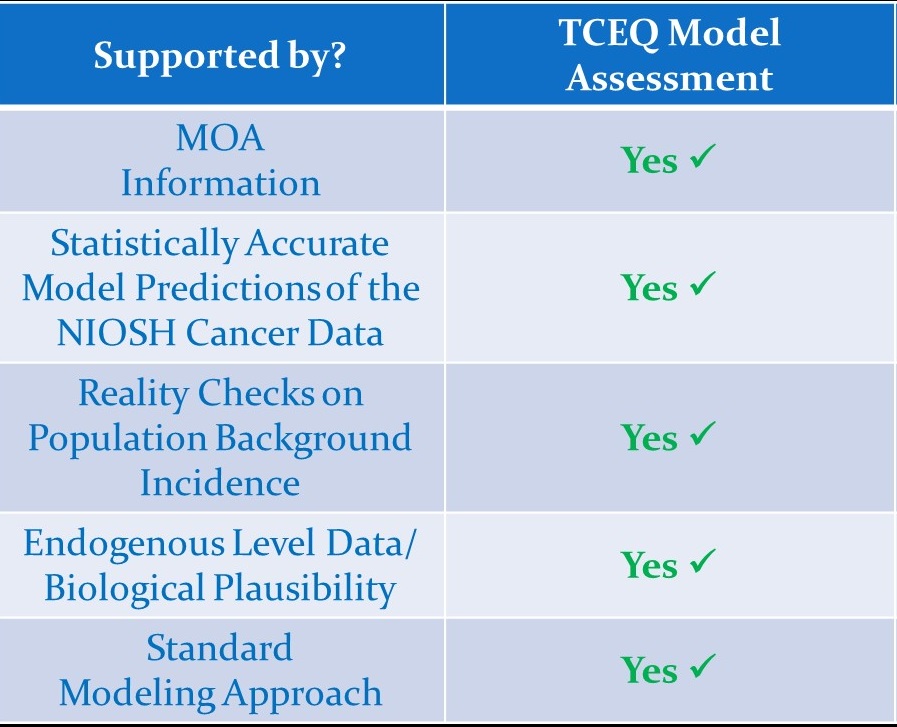
Consider, for example, FDA’s October 25, 2019 statement:
In light of the possibility of continued EtO sterilization facility closures, FDA is again alerting the public to growing concerns about the future availability of sterile medical devices and impending medical device shortages.
More than 20 billion devices sold in the U.S. every year are sterilized with EtO, accounting for ≈50% of devices that require sterilization.
Without adequate availability of EtO sterilization, FDA anticipates a national shortage of critical devices.
In short: this method is critical to our health care system and to the continued availability of safe, effective and high-quality medical devices.
The impact resulting from closure of facilities will be difficult to reverse, and ultimately could result in years of spot or nationwide shortages of critical medical devices, which could compromise patient care.
All this emphasizes the critical importance of scientific scrutiny of EtO dose-response assessments to help ensure the use of best available science for EtO risk assessment.
- FDA: Ethylene Oxide Sterilization for Medical Devices
- FDA: Statement on concerns with medical device availability due to certain sterilization facility closures
- EPA: Ethylene Oxide Data Summary from National Air Toxics Trends Stations and Urban Air Toxics Monitoring Program site
- EPA: Reconsideration of the 2020 National Emission Standards for Hazardous Air Pollutants: Miscellaneous Organic Chemical Manufacturing Residual Risk and Technology Review

