Operational Evaluation Requirements
Public water systems on quarterly monitoring schedules for disinfection byproducts must comply with operational evaluation requirements.
The Operational Evaluation is intended for water systems to take proactive steps to assess source water, treatment, and distribution system processes to decrease high disinfection byproduct (DBP) levels and avoid maximum contaminant level (MCL) violations. The evaluation must be completed when your system has an exceedance of the Operational Evaluation Level. The Operational Evaluation Report is used to document and assist systems through the Operational Evaluation. Disinfection Byproducts in Public Water Systems (PWS) webpage has more information related to DBPs.
On this Page:
- Calculating the Operational Evaluation Level
- Operational Evaluation Report
- Failure to Submit an Operational Evaluation Report
- Factors that Influence DBP Formation
- Controlling DBP Levels
Calculating the Operational Evaluation Level
The Operational Evaluation Level (OEL) is a projected average using Total Trihalomethane (TTHM) and Haloacetic Acids (HAA5) results at each monitoring location and compared against the MCL. Results for the two previous quarters (PQ1 and PQ2) plus twice the current quarter (CQ) result divided by 4.
If the OEL is over the MCL for either TTHM or HAA5 at any monitoring location, it is an OEL exceedance. An OEL exceedance triggers the requirement that your system must perform an Operational Evaluation and submit an Operational Evaluation Report to TCEQ.
The OEL is calculated differently than the Locational Running Annual Average (LRAA), which is used for MCL Compliance. The LRAA is calculated using four running quarters (Q) divided by 4 for both TTHM and HAA5 at each monitoring location.
OEL formula: (PQ1 + PQ2 + CQ + CQ) / 4 = OEL
- If the OEL for TTHM is more than 80 µg/L then you have an OEL exceedance.
- If the OEL for HAA5 is more than 60 µg/L then you have an OEL exceedance.
LRAA formula: (Q1 + Q2 + Q3 + Q4) / 4 = LRAA
- If the LRAA for TTHM is more than 80 µg/L then you have an MCL Violation.
- If the LRAA for HAA5 is more than 60 µg/L then you have an MCL Violation.
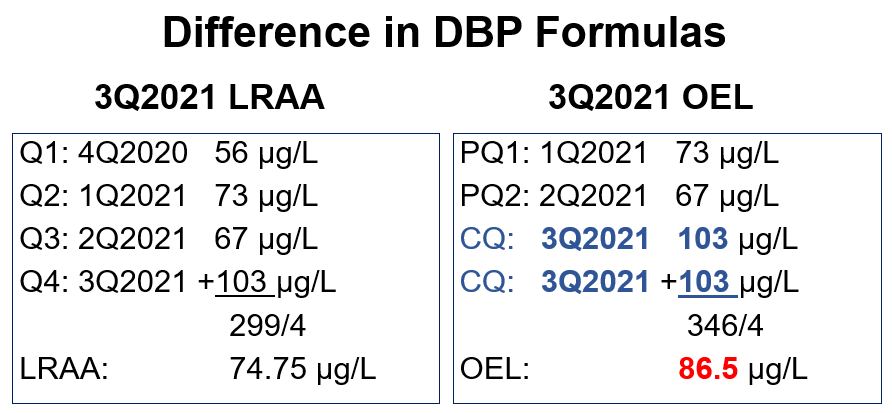
Example data set for TTHM:
In the example below, a system calculates both the LRAA and the OEL for TTHM at one monitoring location. The LRAA is under the MCL, thus the system did not receive an MCL violation. However, the calculated TTHM OEL of 86.5 µg/L is greater than 80 µg/L and is an OEL exceedance.
Operational Evaluation Report
In the event of an OEL exceedance, water systems must conduct an operational evaluation and submit the Operational Evaluation Report to TCEQ. The Operational Evaluation Report is an evaluation of treatment and distribution at the time of the OEL exceedance. The TCEQ Operational Evaluation Report forms walks systems through an Operational Evaluation for the different drinking water source types across Texas.
Please choose the report form that matches the system’s State Primary Source Water Type.
- TCEQ Surface Water (SW) and Groundwater Under the Influence of Surface Water (GUI) Operational Evaluation Report ( TCEQ-20797a)
- TCEQ Groundwater (GW) Operational Evaluation Report ( TCEQ-20797b)
- TCEQ Surface Water Purchase (SWP) and Groundwater Purchase (GWP) Operational Evaluation Report ( TCEQ-20797c) Coming Soon
The system must submit the report to TCEQ within 90 days after the exceedance has been identified by either the system receiving DBP results from the lab or notification from TCEQ of the OEL exceedance, whichever comes first. You can use any of the below methods to submit an Operational Evaluation Report to TCEQ.
Email: DBP@tceq.texas.gov
Mail: TCEQ Drinking Water Standards Section
MC-155 Attn: DBP
PO Box 13087
Austin TX 78711-3087
Failure to Submit an Operational Evaluation Report
An OEL exceedance itself is not a violation and does not require a public notification, but failure to submit a complete Operational Evaluation Report within 90 days of identification of the OEL exceedance will result in a monitoring and reporting (M/R) violation and require public notification.
A maximum contaminant level violation can occur in the same quarter as an OEL exceedance. DBP MCL compliance is under the same rule, however, is calculated using the LRAA as described above under Calculating the Operational Evaluation Level.
Factors that Influence DBP Formation
By considering conditions that favor DBP formation, public water systems can work to achieve lower DBP levels. Water systems can effectively balance disinfection and limit disinfection byproduct formation. Factors influencing DBP formation:
- Concentration of precursors - DBPs can be formed by natural organic and inorganic material in the source water interacting with disinfectant. Systems can reduce total organic carbon (TOC) with coagulation or filtration can reduce DBP levels. Bromides can be reduced by reverse osmosis (RO) filtration.
- Disinfectant type or dose –
- Free chlorine produces the most DBPs.
- Chloramines produce less and are effective at reducing DBP formation especially when bromides are present. A Nitrification Action Plan is required for all systems using chloramines.
- Chlorine Dioxide produce less TTHM and HAA5 but can produce chlorite.
- Ozone does not produce any TTHMs or HAA5s but can produce Bromate when bromides are present.
- Water temperature - the chemical reactions that form DBPs are sped up by warmer temperatures, therefore DBP levels tend to increase in the warmer spring and summer months.
- Disinfectant contact time - the longer water sits in the distribution system, the more time there is for DBPs to form.
- Water chemistry - formation of TTHMs in relation to HAA5s change with pH. HAA5 is more likely to form at low pH, and TTHM is more likely to form at high pH.
- Bromide - elevated levels can lead to increased formation of brominated DBPs, especially when free chlorine is being used.
Controlling DBP Levels
There are many options available for treating or preventing DBPs that vary in cost and complexity. Your strategy to solve the issue should identify the cause of the high DBPs and then target those factors. The table below discusses some but not all treatment options and causes of high DBPs.
Cause of High DBPs
|
Treatment Options |
Precursors (Bromide/TOC) |
Disinfectant (Free/ Total) |
Contact Time (Water age/ Treatment) |
Chemistry (Brominated/ Chlorinated) |
|
Change or Blend Water Source |
Both |
|
|
Both |
|
Modify Reservoir Operations |
TOC |
|
|
Chlorinated |
|
Eliminate Raw Water Chlorination |
Both |
Free |
Treatment |
Both |
|
Optimize Disinfectant Dose |
Both |
|
Both |
|
|
Utilize or Optimize Powdered Activated Carbon (PAC) |
TOC |
|
|
Chlorinated |
|
Optimize/Change Current Coagulation Method |
TOC |
|
|
Chlorinated |
|
Change Disinfectant |
Bromide |
Free |
Treatment |
Brominated |
|
Implement Post Filtration Granular Activated Carbon (GAC) |
TOC |
|
|
Chlorinated |
|
Implement Reverse Osmosis (RO) Treatment |
Both |
|
|
Both |
|
Implement Membrane Treatment |
TOC |
|
|
Chlorinated |
|
Optimize Distribution System Chlorination |
|
Both |
Water Age |
Both |
|
Change Secondary Disinfectant |
Both |
Both |
Treatment |
Both |
|
Optimize Distribution System/Tank Operation |
|
|
Water Age |
Chlorinated |
|
Flush System |
|
|
Water Age |
Chlorinated |
Not every option is feasible for every public water system, but many are relatively simple. Disinfectant levels must meet disinfection standards, but there is often room to optimize the dose. Water systems can also change the type of disinfectant. Chloramines, chlorine dioxide, and ozone will form fewer DBPs, but require other considerations. Precursors can be reduced by coagulation and filtration. If coagulation is already completed, its performance may be optimized by adjusting dose, methods, or changing coagulants. Activated carbon can also be used to remove precursors. If a system has bromide as a precursor, Reverse Osmosis (RO) filtration could be another option for treatment.
Typically, higher DBP levels are found in areas of the distribution with the highest water age (the oldest water). Systems should try to decrease the amount of time finished water remains in storage and in the distribution system. Periodic flushing of sections of the distribution system prone to long retention times can reduce DBP levels.
DBP reduction must be balanced with disinfection, corrosion control, and other requirements. For instance, changes in pH to treat for TTHMs could corrode pipes and create lead and copper issues while also generating HAA5s. Insufficient chlorine residuals could leave a system vulnerable to pathogenic organisms and nitrification. As with any change in water operations, it is essential to evaluate compliance with other measures simultaneously.
- If you need assistance completing the evaluation, or for help navigating the website contact the TCEQ DBP compliance coordinator at (512) 239-4691 or email DBP@tceq.texas.gov.
- To request free assistance for your DBP issues contact the Financial, Managerial, and Technical Assistance program at (512) 239-4691 or FMT@tceq.texas.gov.
To view your system's DBP results, visit Drinking Water Watch.
